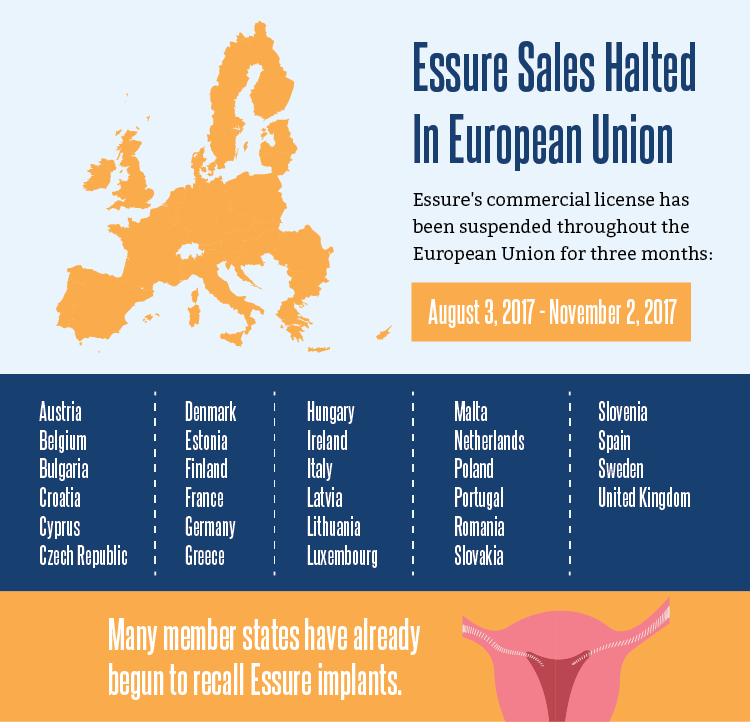
Despite Bayer’s strident claims to the contrary, regulators in Ireland aren’t exactly certain the German company’s Essure “permanent” birth control device is a safe option. That uncertainty prompted the National Standards Authority of Ireland (NSAI) to decline renewal of Essure’s CE marking – the commercial license that allows the device to be sold in the EU.
Despite Bayer’s strident claims to the contrary, regulators in Ireland aren’t exactly certain the German company’s Essure “permanent” birth control device is a safe option. That uncertainty prompted the National Standards Authority of Ireland (NSAI) to decline renewal of Essure’s CE marking – the commercial license that allows the device to be sold in the EU.
According to the European Commission’s website:
“The letters ‘CE’ appear on many products traded on the extended Single Market in the European Economic Area (EEA). They signify that products sold in the EEA have been assessed to meet high safety, health, and environmental protection requirements. When you buy a new phone, a teddy bear, or a TV within the EEA, you can find the CE mark on them. CE marking also supports fair competition by holding all companies accountable to the same rules.”
What this means in concrete terms is that Bayer cannot legally sell Essure in the European Union during the three-month suspension of its CE marking. Nor can healthcare providers and hospitals legally implant the device. The CE marking expired on August 3, and may be renewed on November 2, 2017 pending review by the NSAI. If the Irish agency doesn’t see a favorable conclusion of the review, Essure will no longer be available in the EU.
On a side note, Bayer has already stopped (or plans to stop) sales and distribution of the device in Canada, the UK, Finland, and the Netherlands, citing “business reasons.” This can be translated as declining sales, most likely due to the widespread coverage of the problems associated with Essure. Brazil had banned the device in February of this year, waiting on the results of further testing. Their public health agency recently lifted that ban.

The way the CE marking system works is that companies may choose which EU member country’s regulatory agency handles licensing applications and renewals. Ironically, Bayer chose Ireland and the NSAI. One can assume the company regrets this decision given the NSAI’s actions.
Bayer, as always, is sticking to its canned response to any challenge to Essure’s safety and efficacy. The company’s statement reads, “We would like to reassure the women who use Essure as their form of contraception and their healthcare professionals that the safety and efficacy of Essure continues to be supported by more than a decade of science and real world clinical experience.”
Never mind the tens of thousands of women seriously injured by the device. Bayer, and its “real world clinical experience,” seems to be awfully selective in data it acknowledges.
France’s health agency, the ANSM, put together a group of experts in April of 2017 with the express purpose of reviewing available medical literature pertaining to Essure’s safety. This group mainly focused on a 100,000-woman epidemiological study and decided that data did not support an Essure recall. According to Birth Control Problems, the French authorities “believe that Ireland’s temporary suspension of the device is unlikely to call the committee’s ultimate findings into question.” That remains to be seen, of course.
In the meantime, the ANSM issued a precautionary statement urging women to avoid Essure. It also advised women who have Essure to check with their healthcare providers about possible alternatives to the device. According the Birth Control Problems, “health professionals have been instructed not to implant Essure in new patients.” The Ministry of Healthcare in Ukraine took a different approach, according to The Siver Times. That agency has asked Bayer to recall “all Essure units currently stocked by the country’s healthcare providers.”
Interestingly – and frighteningly – some E-Sisters in the U.K. have been surprised by some area hospitals’ seeming lack of knowledge of the CE marking suspension. In private discussions with two of them, I learned that only a couple hospitals confirmed knowledge of the suspension. More than one had no clue about it or the fact that implantations should not be done during the suspension. One outright stated in email that, “We will continue to offer this procedure until the stores are depleted after which we will be offering laparoscopic sterilisation.”
If you’re confused as to how healthcare providers such as major hospitals are unaware of the CE mark suspension and/or refusing to stop implanting Essure, you are not alone. The members of a private Facebook support group are equally confused. And, they’re demanding answers. So far, all they’ve received is the equivalent of “We understand your concern and will look into it.” Failure to comply with the suspension of implantation violates local laws, according to legal counsel for these women.
The U.K.’s Medicines & Healthcare Products Regulatory Agency (MHRA) seems equally confused. When contacted by the U.K. E-Sisters, the Agency seemed unaware of the suspension, too. An excerpt from their response follows:
“We will share details of the device and what happened with the manufacturer of the device because under European law they are responsible for monitoring the safety of their devices and need to know about any problems with them.”
It’s as though they have no idea that the CE marking was suspended. This prompted the E-Sisters to send a group letter to the Agency just today. There have to be answers. The U.S. FDA isn’t all that concerned with protecting women here, but this is different. In the EU, there is a legal suspension of Bayer’s privilege to sell Essure. There is a legal suspension of all implantation procedures. Why aren’t the proper agencies stepping up and making sure that hospitals not only know about the suspension, but adhere to its rules?
As always, more to come as soon as information is available.

Join the conversation!