7/27/2015

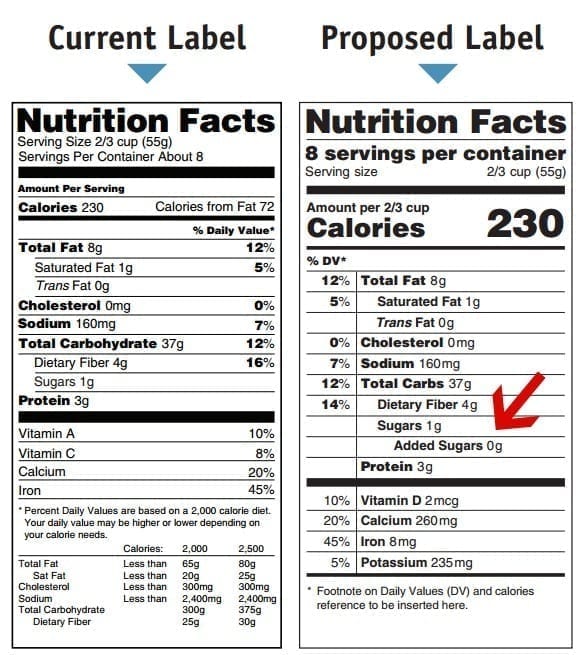
In a move opposed by many food and drink manufacturers, the Food and Drug Administration (FDA) issued a proposal on Friday to requiring the industry to include detailed added sugar consumption levels in their products. The proposal is an update to the FDA’s 2014 proposed mandate requiring companies to list the amount of added sugars in grams; however the new proposal also requires the inclusion of the percentage of daily recommended added sugar intake as well. “Added sugars” is defined as sugar that is not already in the product prior to production and packaging. A February report from the U.S. Dietary Guidelines Advisory Committee recommended that people should not consume more than 10 percent of their daily caloric intake on added sugars. The 2014 proposal was part of the Obama administration’s broad overhaul of the “Nutrition Facts Label,” that had remained unchanged for 20 years. The FDA recommends that adults and children four years-old and older consume no more than 50 grams of added sugar per day, and children 3 or younger no more than 25 grams. The new recommendations would mean that the average adult should not consume more than 200 calories worth of added sugar in a day, or about the equivalent of a 16oz Coca-Cola.
Susan Mayne, the head of the FDA’s Center for Food Safety and Applied Nutrition, explained the rationale behind the proposal writing in a blog post on the FDA’s website, “For the past decade, consumers have been advised to reduce their intake of added sugars, and the proposed percent daily value for added sugars on the Nutrition Facts label is intended to help consumers follow that advice.” Mayne noted that “Scientific data shows that it is difficult to meet nutrient needs while staying within calorie requirements if you consume more than 10 percent of your total daily calories from added sugar.” In addition, Mayne cited that studies show that reduced sugar intake can lead to improved cardiovascular health. The FDA currently places daily recommendations for saturated fat, sodium, cholesterol, and total fat, among others. The proposal accompanies last month’s determination that partially hydrogenated oils, the main source of trans-fats were not “Generally Recognized as Safe” (GRAS), and that food and beverage makers need to eliminate them from the American food supply within three years.
Not surprisingly, the proposal has been met with fierce opposition from food and beverage industry groups. The Sugar Association released a statement saying that the proposal was based on “limited and weak scientific evidence.” They also wrote, “The fact is that the preponderance of science and the data on caloric sweeteners do not support a suggested limit on sugars intake,” saying that the group will “oppose this proposal and examine the level of scientific evidence at the basis of the misguided recommendation.” Six major trade groups, including the American Beverage Association, the American Frozen Foods Institute, and the Corn Refiners Association, among others, penned a letter last month to Michael R. Taylor, the deputy commissioner of the agency’s Office of Foods and Veterinary Medicine, offering instead to fund their own independent research studies as opposed to relying on government sources. Another group funded primarily by the food industry, The International Food Information Council Foundation, published a peer-reviewed study in the Journal of the Academy of Nutrition and Dietetics, arguing that consumers may become confused by the new labeling requirements.
Public health attorney and food-industry critic Michele Simon, however, calls these opposition efforts “a smokescreen.” Simon believes that the industry is “worried consumers will see there’s all this sugar in food that they didn’t know about, and will make decisions accordingly. This will finally give consumers the information they need to make more informed choices.” Both the World Health Organization and the American Heart Association have adamantly warned the public regarding excessive sugar intake’s link to ailments like diabetes and heart disease. Many believe that the American public will be shocked to find out how much sugar they actually consume in packaged foods and beverages compared to the recommended amount. The FDA has opened up the most recent proposal to a 75-day public commenting period prior to making its final determination. The agency also reinstituted a 60-day commenting period for the original 2014 proposal as well. Revision of the proposals could still be possible depending on the amount of support or backlash from the public.
Sources:
Tech Times – Jim Alger
Time – Alexandra Sifferlin
Washington Post – Brady Dennis
Wall Street Journal – Ilan Brat and Mike Esterl

Join the conversation!