Biosensor Inc. is recalling certain at-home COVID-19 tests that may have been illegally imported into the U.S.
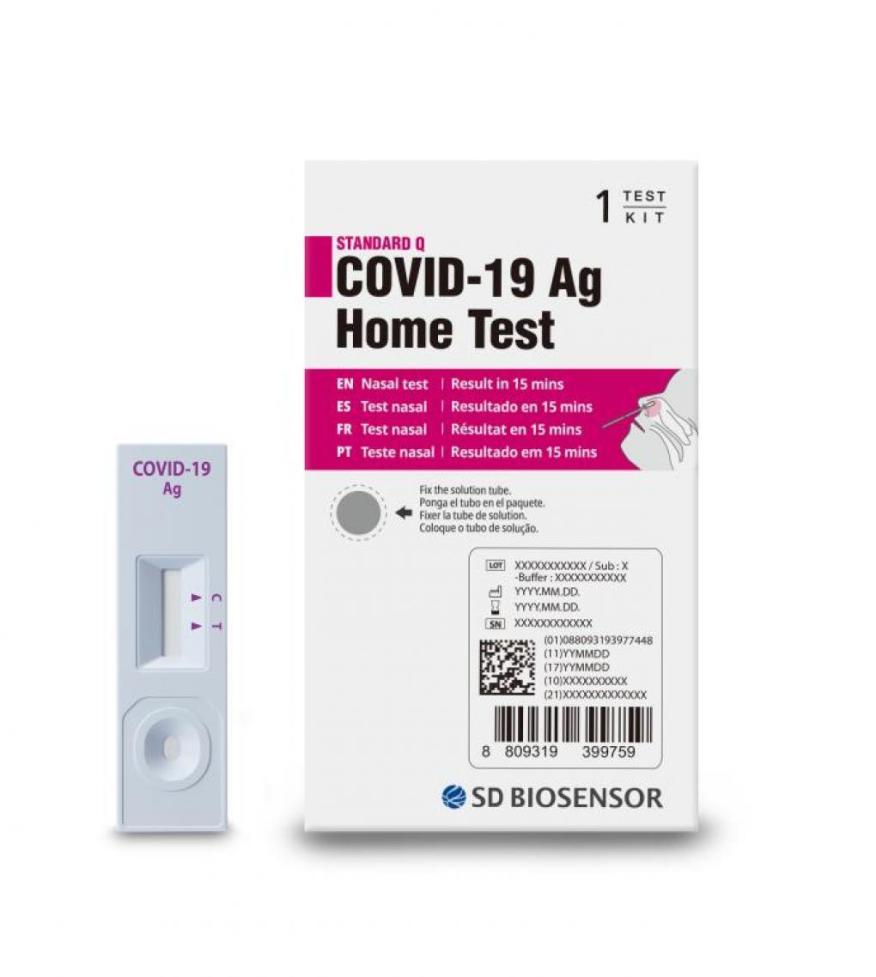
Earlier this week, the U.S. Food and Drug Administration (FDA) issued a recall for certain at-home COVID-19 tests over concerns that they were allegedly imported into the U.S. The tests were manufactured by SD Biosensor Inc., a South Korean diagnostics company. The tests included in the recall are the ‘STANDARD Q COVID-19 Ag Home Test.’

According to the recall notice, the FDA has received “confirmed reports that the test kits were illegally imported into the United States.” The notice further states that the tests “are not authorized, cleared, or approved by the FDA for distribution or use in the U.S.” The notice also states:
“In the unlikely event that consumers in the United States encounter the ‘STANDARD Q COVID-19 Ag Home Test,’ they are encouraged to discard and avoid any use of the test, as it has not been authorized, cleared or approved by the FDA for use in COVID-19 testing and diagnosis in the United States…Consumers that have used the test are strongly encouraged to consider retesting with an FDA authorized or cleared test.”
In addition to the recall, SD Biosensor is also conducting an investigation to find out how the “tests were illegally imported” into the country. On top of that, the company is “taking appropriate measures to prevent further attempts at illegal importation of unauthorized tests by strengthening contract terms and their enforcement with its distributors.” The notice further states:
“In addition, the company announced publicly that if such illegal importations are discovered in the future, the responsible individuals/distributors will face strict legal action and liabilities for damages.”

Join the conversation!