6/24/2015
Two reports released on Tuesday highlight the persistence of Medicare fraud, especially involving the Part D prescription drug program. The reports follow the largest Healthcare Fraud takedown in history last week, as officials charged 46 doctors and pharmacy owners, as well as nearly 200 others for Medicare fraud. This includes the arrest of 44 healthcare professionals for Part D fraud. The nationwide sweep has led to prosecutors charging those cited for $712 million of questionable billing. Both reports were issued by the Health and Human Services’ Office of the Inspector General (OIG). The first report cites at least 1,400 pharmacies that engage in questionable billing practices for opioid medications. The second report calls for the Centers for Medicare and Medicaid Services (CMS) to implement more of the OIG’s recommendations for preventing Medicare fraud. Medicare Part D is the fastest growing component of Medicare, covering 39 million people to the tune of $121 Billion in 2014. In 2006, the amount was only $53 billion.
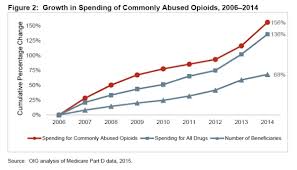
The first OIG report notes that over 1,400 pharmacies have exceptionally high numbers of opioid prescriptions per patient, as well as an abnormal proportion of prescriptions of narcotic controlled substances. The 1,400 pharmacies account for $2.3 billion in Medicare reimbursements in 2014. The report also notes that Medicare billing for prescription opioids has increased from $1.5 billion to $3.9 billion from 2006 to 2014, a 156 percent increase, whereas Part D billing overall has only increased by 136 percent. It also identified specific states, Alabama, Tennessee, Oklahoma and Alaska, where over 40 percent of beneficiaries filled at least one narcotic prescription compared to the national average of 32 percent. The report also cites specific metro areas like New York and Los Angeles to be rife with fraud, especially among rare and expensive medications that have been associated with fraud in the past. Among these is the topical skin ointment, Solaraze, as well as the drugs, Vascepa and Lovansa. The OIG has criticized CMS on multiple occasions for its failure to identify widespread fraud, although the department is credited for taking some steps towards closer scrutiny. An inspector for the New York office, Jodi Nudelman, said “CMS has made progress on a number of recommendations we’ve made, as well as on the initiatives that they’ve had. They’re starting to use data to drive their strategies.”
Despite some progress being made, the second report stresses the need for CMS to adopt a series of changes that the agency has so far resisted. Among the reforms includes mandating that health plans report instances of fraud to CMS or its enforcement contractors. Currently, the reporting is voluntary, with OIG concluding that only about half of the insurance providers regularly report such instances. Also the report suggests that CMS should expand its reviewing procedures beyond just for controlled substances, including reviews for antipsychotic drugs, as well as respiratory and HIV medications. The Inspector General also recommends that CMS obtains the legal authority to restrict the amount of health professionals that beneficiaries suspected of “doctor-shopping” can choose. Additionally, the report recommends that CMS find a method to reject prescriptions filled by excluded providers as well as review health plans’ fraud prevention systems. CMS did grant itself authority to expel pharmacies from Part D if they were suspected of fraudulent behavior, but that authority has been delayed twice, and is now scheduled to begin January 1st. CMS also launched a website in April to allow the agencies, as well as law enforcement and health plans to better monitor suspicious activity. CMS spokesman, Aaron Albright, stressed in a written statement that the agency has worked diligently with law enforcement to prevent fraud and abuse. While Nudelman did give credit for the reforms that CMS has implemented, she stressed, “There are still concerns. More needs to be done. We can’t stop here.”
Sources:
Modern Healthcare – Lisa Schencker
National Law Review – Stanford Moore
NPR – Charles Ornstein


Join the conversation!