Viona Pharmaceuticals is recalling certain batches of diabetes medication that may contain too much NDMA, a probable cancer causing agent.
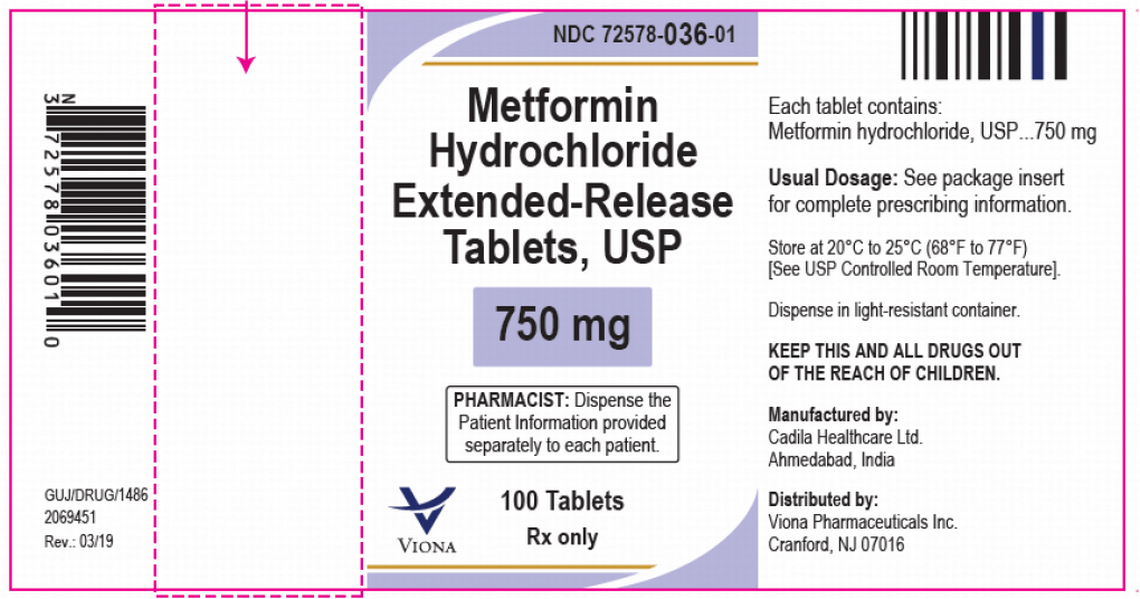
Viona Pharmaceuticals is recalling certain lots of type 2 diabetes drug 750 mg strength metformin over concerns that the drug contains too much NDMA. NDMA, otherwise known as N-nitrosodimethylamine, is a probable human carcinogen and was linked to countless metformin recalls in 2020.

According to the notice, 33 lots are included in the recall. At the moment, only the Metformin Hydrochloride Extended-Release Tablets, USP 750 mg in 100-count bottles are affected. The tablets are off-white in color and have a debossed ‘Z’ or ‘C’ printed on one side of the table and ‘20’ printed on the other side. The following batch numbers are included in the recall:
- M008130-133, exp. date 06/2022
- M010080-81, exp. 07/2022
- M011029-32, exp. 08/2022
- M013394-96, M013966-67, exp. 09/2022
- M100831-32, exp. 12/2022
- M100833-34, M101267, M102718-20, exp. 01/2023
- M102721-22, M104172-76, exp. 02/2023
- M105889-90, exp. 03/2023
It’s important to note that this medication will not give you cancer immediately. Rather, the concern is that it could cause cancer after prolonged use. Because of this, it is being advised that consumers continue taking their medication until instructed not to by their doctor.
At the moment, Viona is working on informing customers of the recall and is arranging a process for returns. If you have additional questions or concerns about the recall, contact Eversana at 888-304-5022, option No. 1, Monday through Friday, 9 a.m. to 8 p.m., Eastern time.
Sources:
Company recalls a diabetes drug because it might have too much of a carcinogen
FDA: Diabetes Medication Recall Due To Cancer Concerns, What Is Metformin?

Join the conversation!